
Till date, our electron matter is 2 x 2 = four.

The antibonding MOs are empty.Īs a end result, we Will see that we have accounted for the σ bonding of the sulfur with every oxygen. The σ bonding MOs are labeled 1 for the left S−O σ bond, and 2 for the right S−O σ bond. Two of the sp 2 AOs (2s+2px+2py) from oxygen can overlap head-on with of the sp 2 AOs (2s+2px+2py) from sulfur to form one σ bonding and one σ*antibonding MO. The 3s and 3p orbitals on sulfur mix to supply hybridized sp 2 AOs for σ bonding (the hybridized orbitals are indicated by means of the "S sp 2 " label at the far left of the diagram) that are intermediate in electricity among the original 3s and 3p AOs.
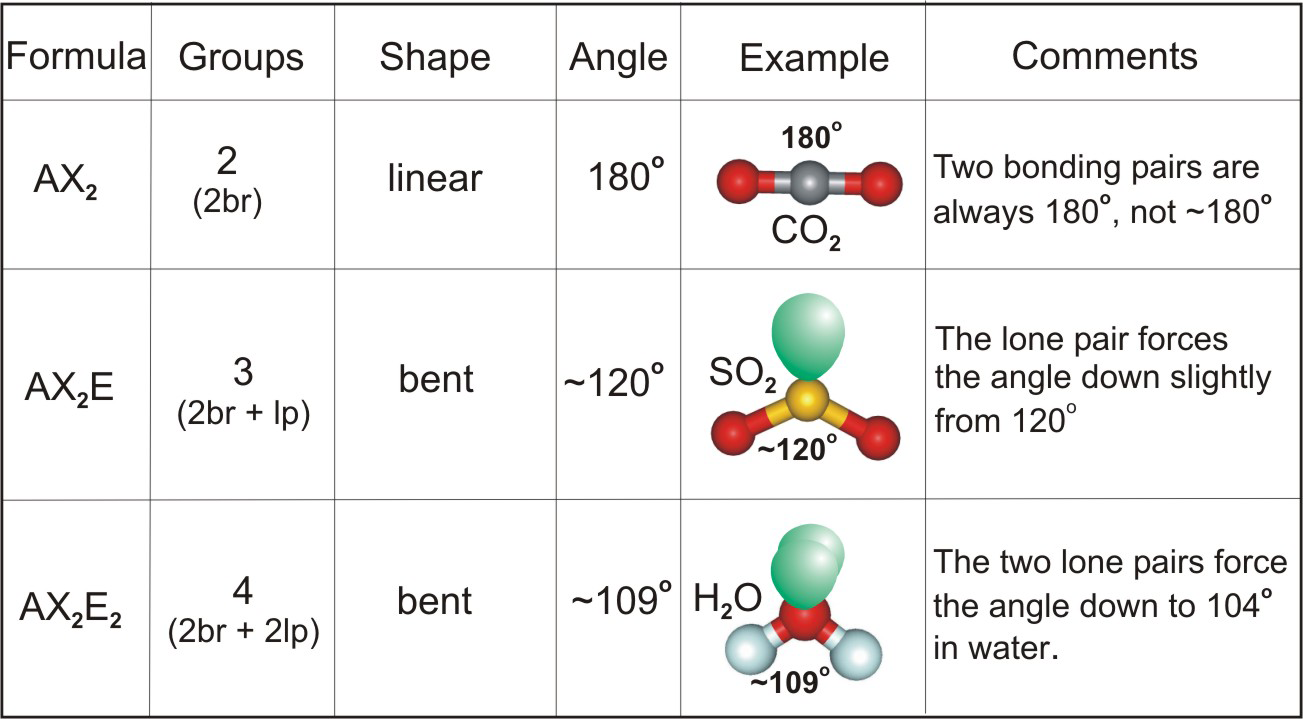
The 2s and 2p orbitals on oxygen mix to produce hybridized sp 2 AOs for σbonding (the hybridized orbitals are indicated through the "O sp 2 " label on the far right of the diagram) which can be intermediate in power between the unique 2s and 2pAOs. Sulfur has one 3s, three 3p, and five three-D valence atomic orbitals (AOs), while oxygen has one 2s and 3 2p valences AOs. SF 6 ), however oxygen can't due to the fact sulfur get right of entry to its 3-d orbitals (it's at the 1/3 period/row) You may then position four electrons on every oxygen and the last 2 at the sulfur as a lone pair. Consequently, 6×three=18 valence electrons distribute in the course of the structure placing 4 for every of two double bonds makes use of up to eight. Sulfur desires 6 electrons, and so does oxygen. SO 2 is a bent shape (molecular geometry). The hybridization of the two oxygen atoms is sp 2 as properly. The two unpaired electrons within the unhybridized orbitals participate in the formation of pi bonds.Ĭonsequently, the hybridization of the principal sulfur atom in this compound is sp 2. The ultimate 3p and 3-D orbitals remain unhybridized.
One hybrid orbital is occupied by means of the lone pair and different two orbitals have unpaired electrons, which take part in sigma bonding with the oxygen atoms. One 3s and 3p orbitals get hybridized to shape three same sp2 hybrid orbitals. Now, there are 4 unpaired electrons (three unpaired electrons in three 3p orbitals and one unpaired electron in one 3-D orbital).Īs the electrons forming sigma bonds (and the lone pair) want to be on an equal energy level, hybridization takes place. Therefore, the formation of the excited state takes place: One 3px electron shifts to an empty 3D orbital. which will form 4 bonds, it desires four unpaired electrons. There are two paired electrons in the 3s orbital and 4 electrons in 3p orbital ( paired electrons in 3px orbital and one unpaired electron each in 3py and 3pz orbitals). Sulfur in its ground state has first shells fully filled and 6 electrons within the outermost shell. The shape is like this: O=S=OThe sulphur atom forms one sigma and one pi bond with every oxygen atom and has one lone pair.

The primary sulphur atom is bonded to two oxygen atoms. considering that there are three electron clouds, then it is SP 2 and it takes a trigonal planar geometry. two from its two double bonds with oxygen, and a lone pair of electrons. The central atom, Sulfur, has 3 electron clouds in this molecule.


 0 kommentar(er)
0 kommentar(er)
